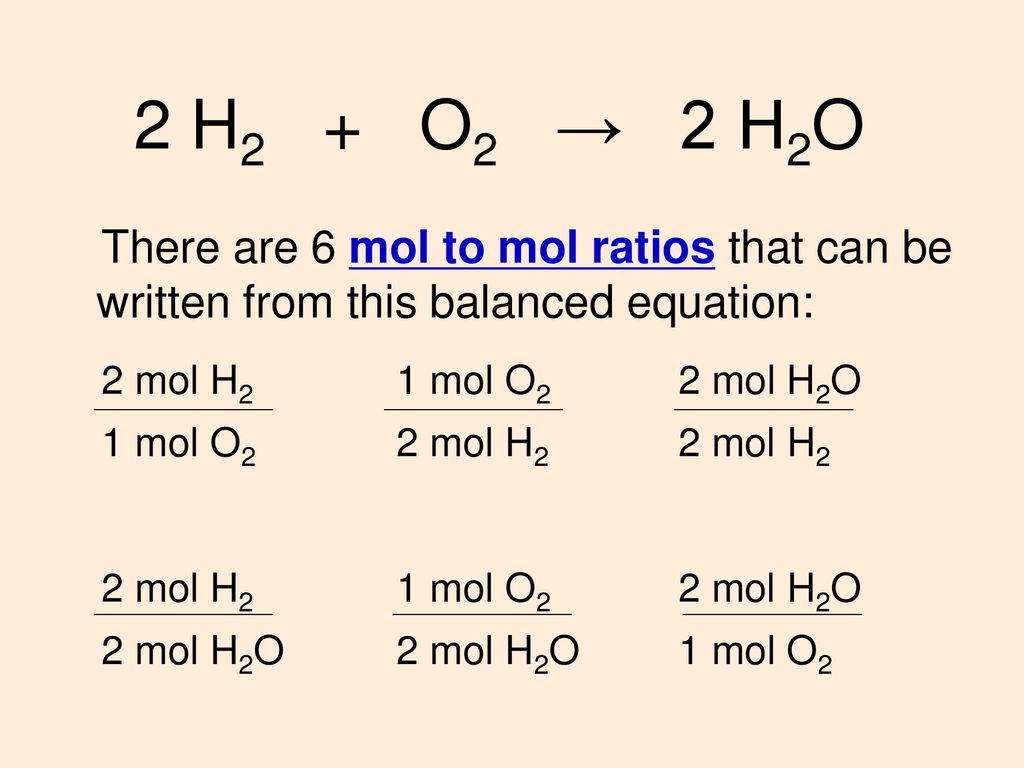
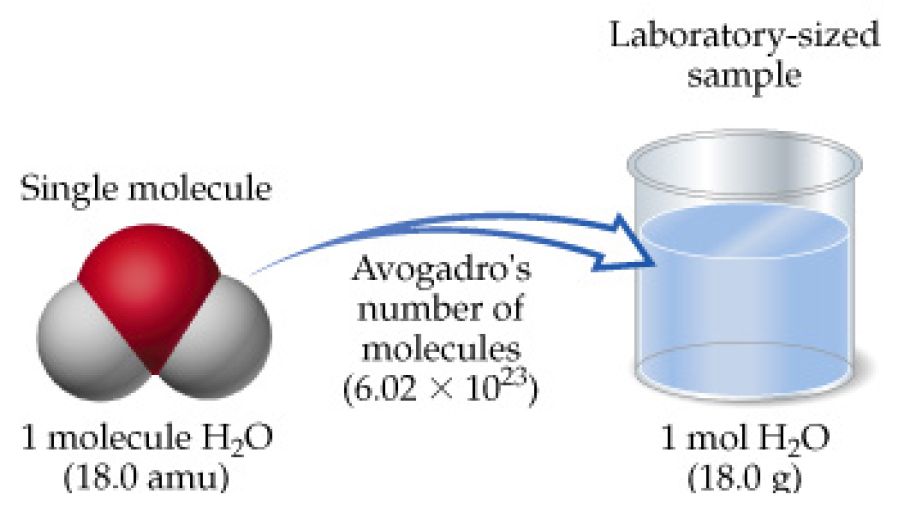
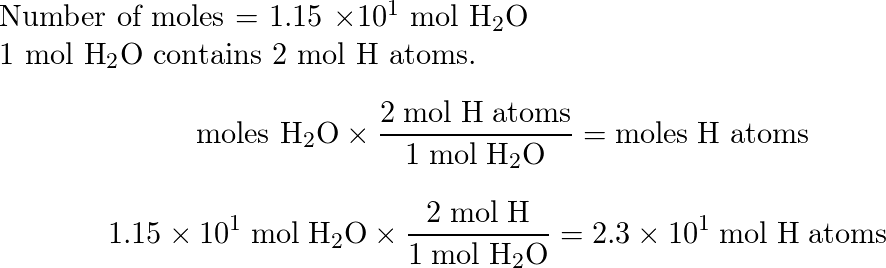![Calculate the depression in the freezing point of water when 10 g of CH3CH2CHClCOOH is added to 250 g of water. [ Ka = 1.4 × 10^-3, Kf = 1.86 K kg mol^-1 ] Calculate the depression in the freezing point of water when 10 g of CH3CH2CHClCOOH is added to 250 g of water. [ Ka = 1.4 × 10^-3, Kf = 1.86 K kg mol^-1 ]](https://d1hhj0t1vdqi7c.cloudfront.net/v1/ekdmSWJoaW9GWjA=/sd/)
Calculate the depression in the freezing point of water when 10 g of CH3CH2CHClCOOH is added to 250 g of water. [ Ka = 1.4 × 10^-3, Kf = 1.86 K kg mol^-1 ]

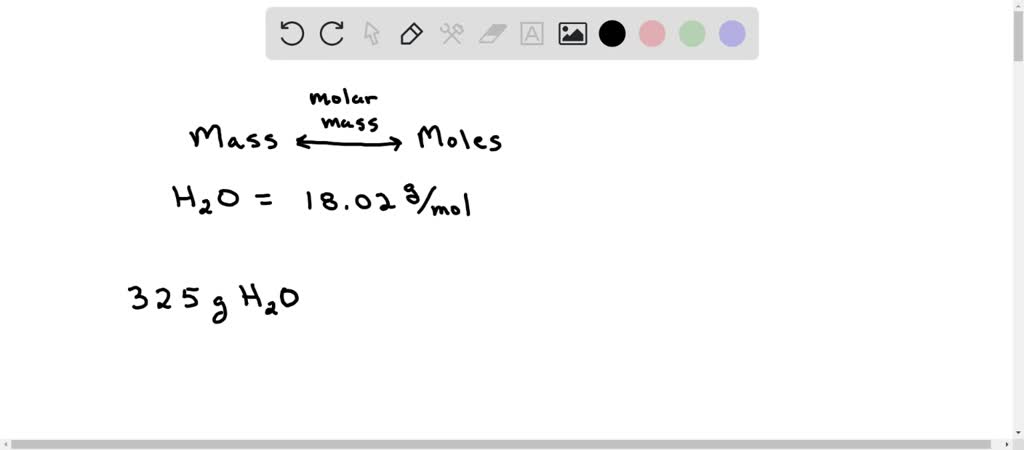
Question Video: Calculating the Mass of Water Produced Given the Masses of Oxygen and Hydrogen | Nagwa

Water solubility in CO2 (H2O mol %) as a function of pressure (MPa) and... | Download Scientific Diagram

Solved: 9 mol P4010 reacts with 51 mol H₂O according to the equation below: P4010 + 6H₂O → 4H3PO4 How many - Brainly.com