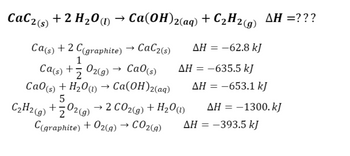
SOLVED: Calcium carbide (CaC2) reacts with water to form acetylene (C2H2): CaC2 (s) + 2 H2O (g) → Ca(OH)2 (s) + C2H2 (g) How many grams of water are required to produce

Phản ứng CaC2 + H2O → C2H2 + Ca(OH)2? Cách tạo khí axetylen từ phản ứng CaC2 + H2O - Cao đẳng Nghề Việt Mỹ
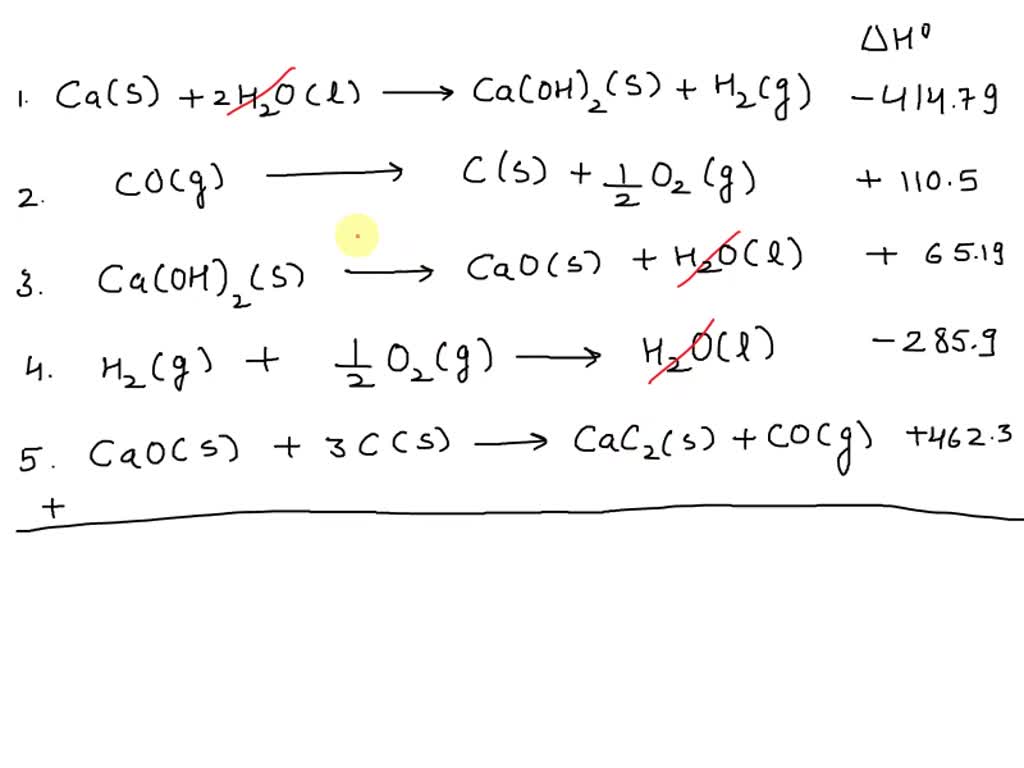
SOLVED: Calculate the standard heat of formation of calcium carbide, CaC2(s), in kJ/mol using the following thermochemical equations. Ca(s) +2H2O(l) 🡪 Ca(OH)2(s) +H2(g) ∆H° = - 414.79 kJ 2C(s) +O2(g) 🡪 2CO(g)

Fluoride-Assisted Activation of Calcium Carbide: A Simple Method for the Ethynylation of Aldehydes and Ketones | Organic Letters
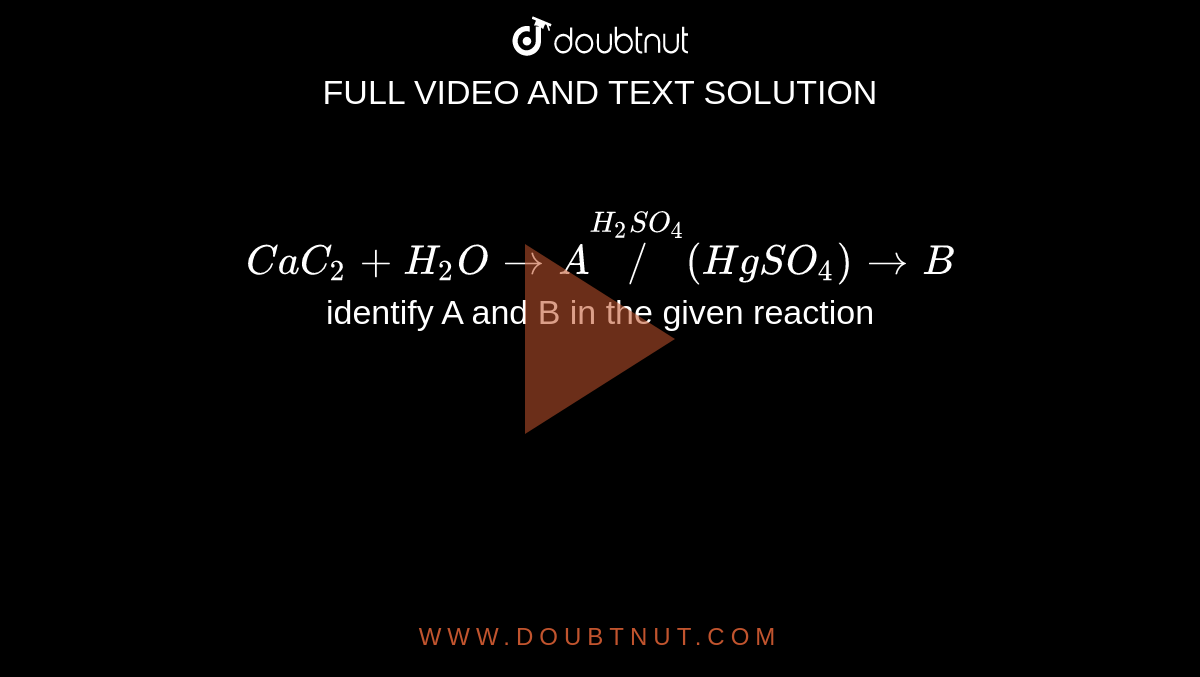
Complete the following reaction and name the products A, B and C. Cac2 + ( H2O) → A + (hot Cu tube) → B + ((conc. H2SO4 + HNO3)/(323 - 333K)) → C - Sarthaks eConnect | Largest Online Education Community
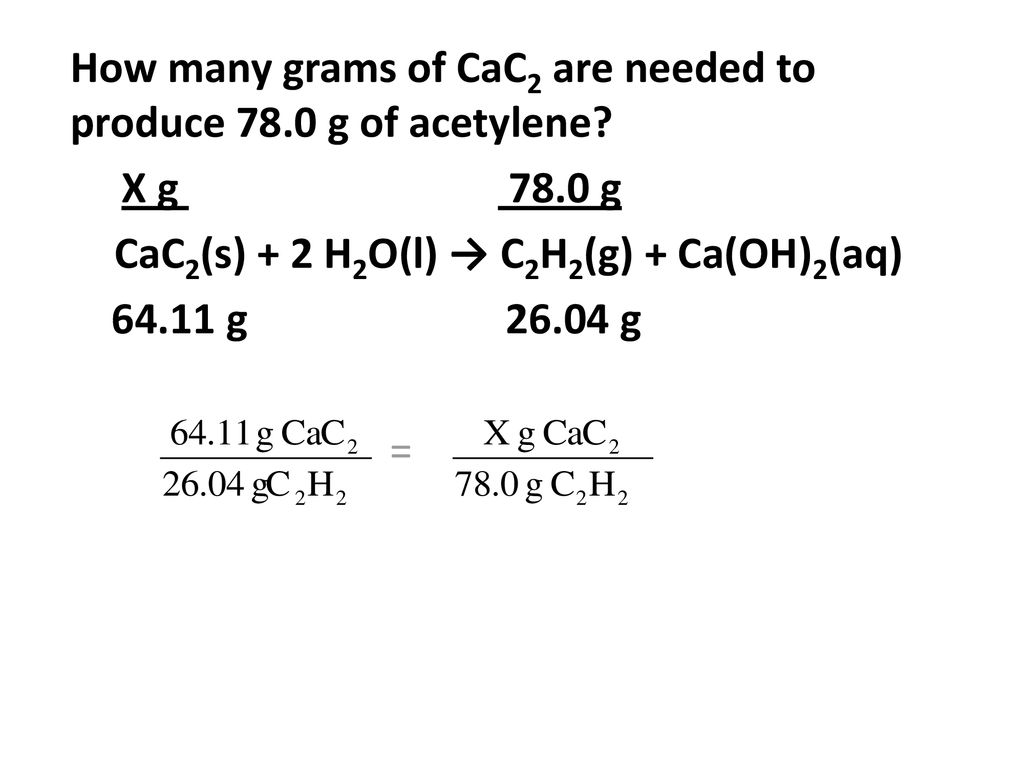
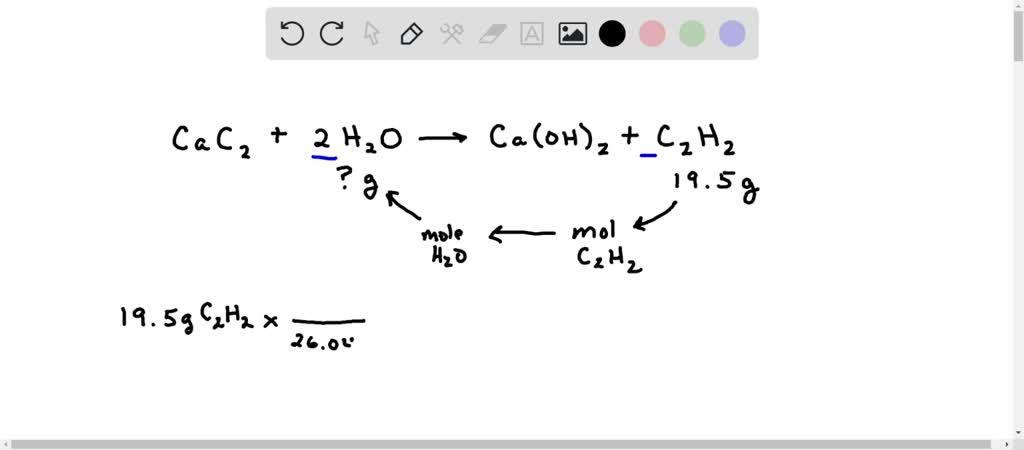
Bell ringer # 2 : How many grams of CaC2 are needed to produce 78.0 g of acetylene? CaC2(s) + 2 H2O(l) → C2H2(g) + Ca(OH)2(aq) TIME'S UP! START TIMER ppt download

Towards C1 chemistry: methanol vinylation by CaC2 in water in the presence of potassium or sodium carbonates - Parshina - 2019 - Journal of Chemical Technology & Biotechnology - Wiley Online Library
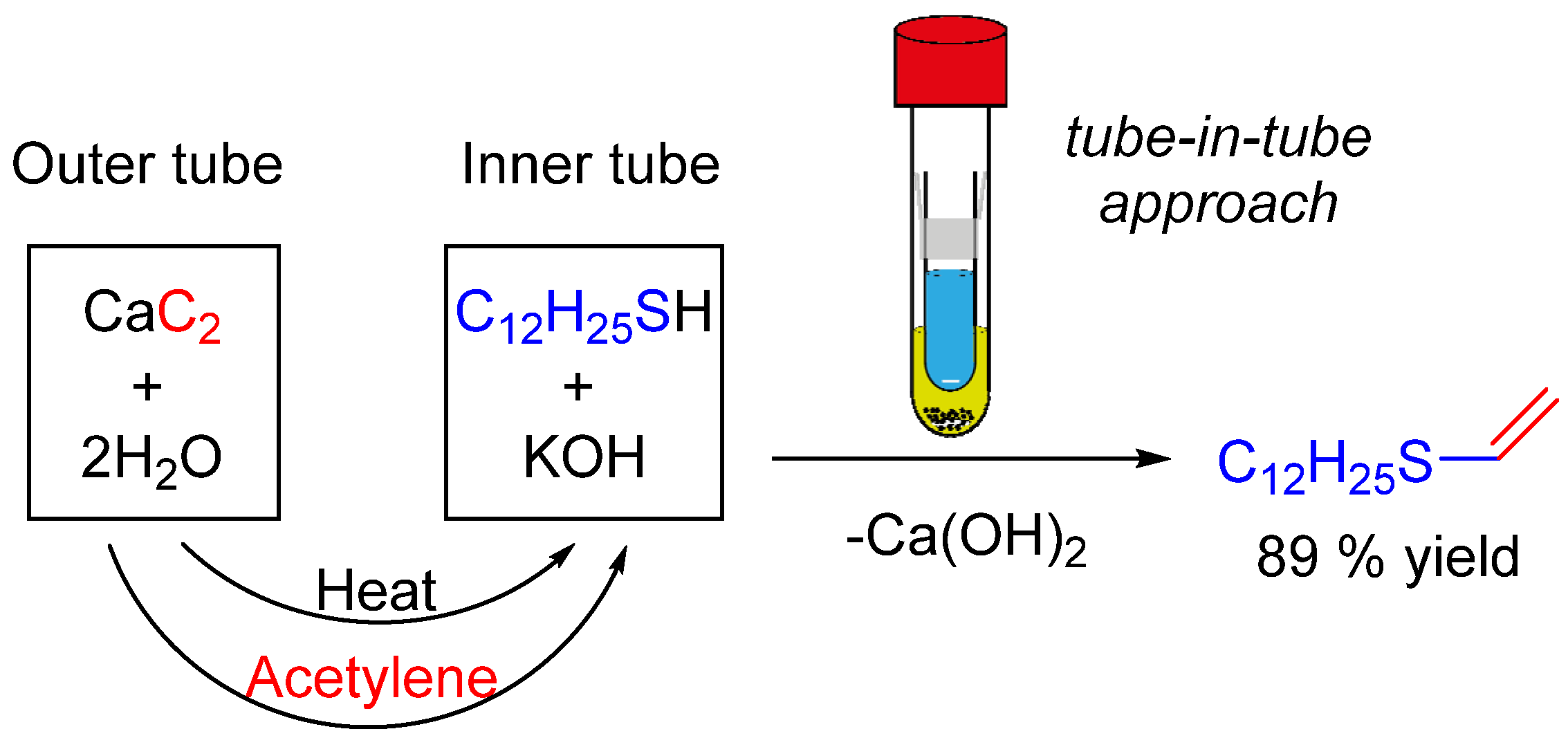
IJMS | Free Full-Text | Thermal Mapping of Self-Promoted Calcium Carbide Reactions for Performing Energy-Economic Processes
Calcium carbide, CaC2, reacts with water to form ethyne, C2H2, and calcium hydroxide. The equation for the reaction is shown. CaC2(s) + 2H2O(l) → C2H2(g) + Ca(OH) 2(s), which volume of ethyne