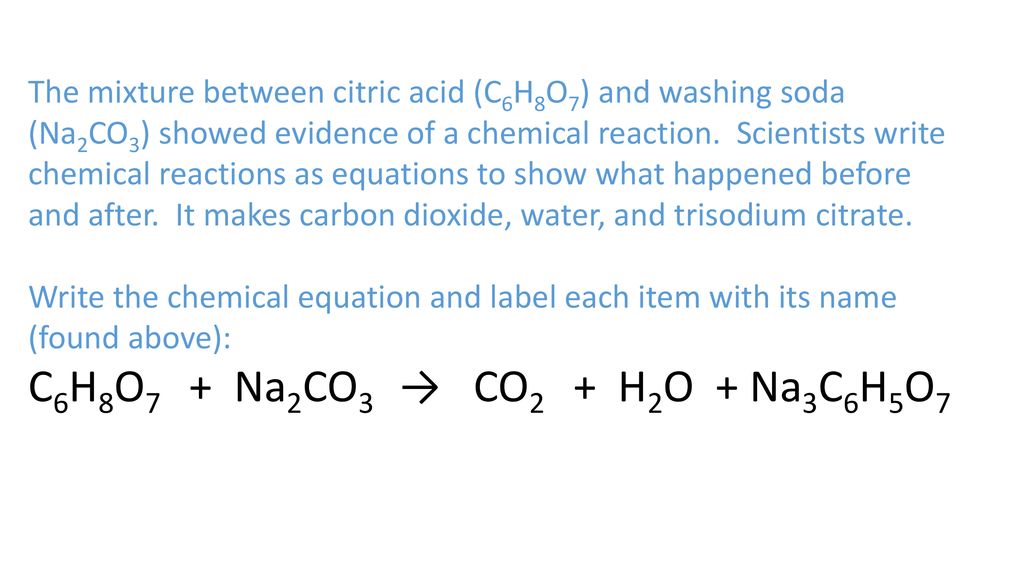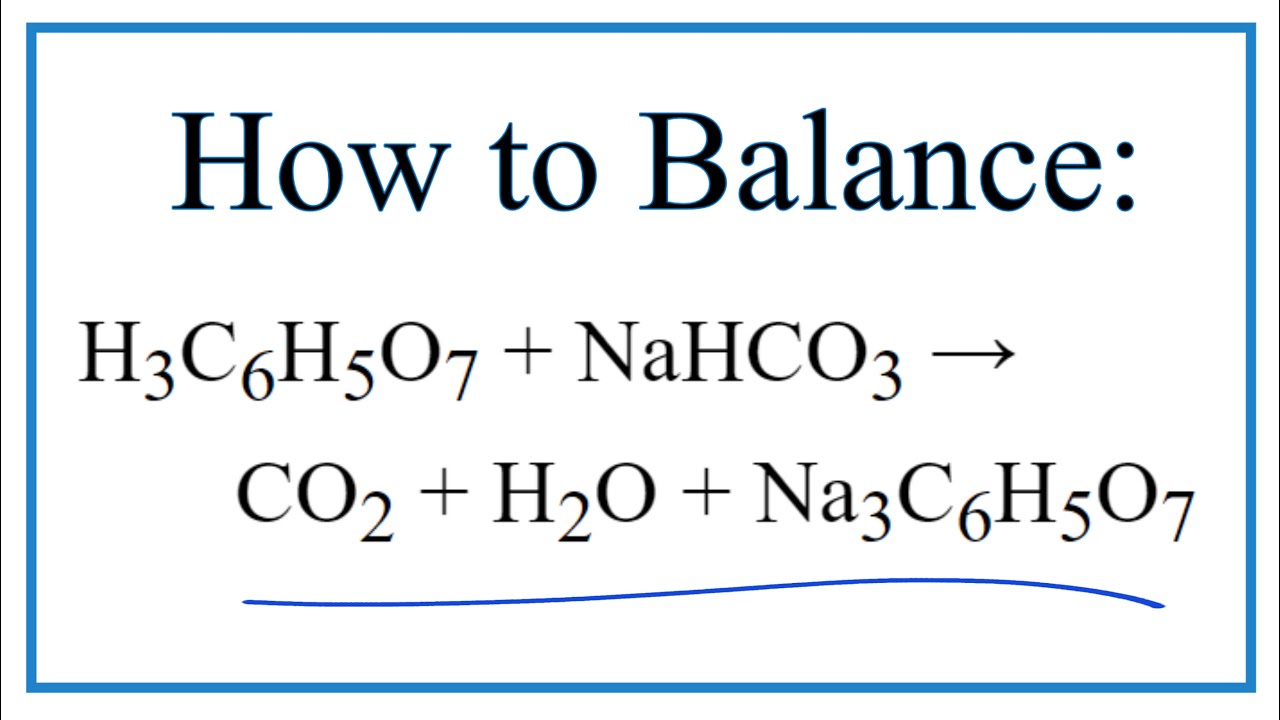
How to Balance H3C6H5O7 + NaHCO3 = CO2 + H2O + Na3C6H5O7 (Citric acid + Sodium bicarbonate ) - YouTube

Structural Diversities of Cobalt(II) Coordination Polymers with Citric Acid | Crystal Growth & Design
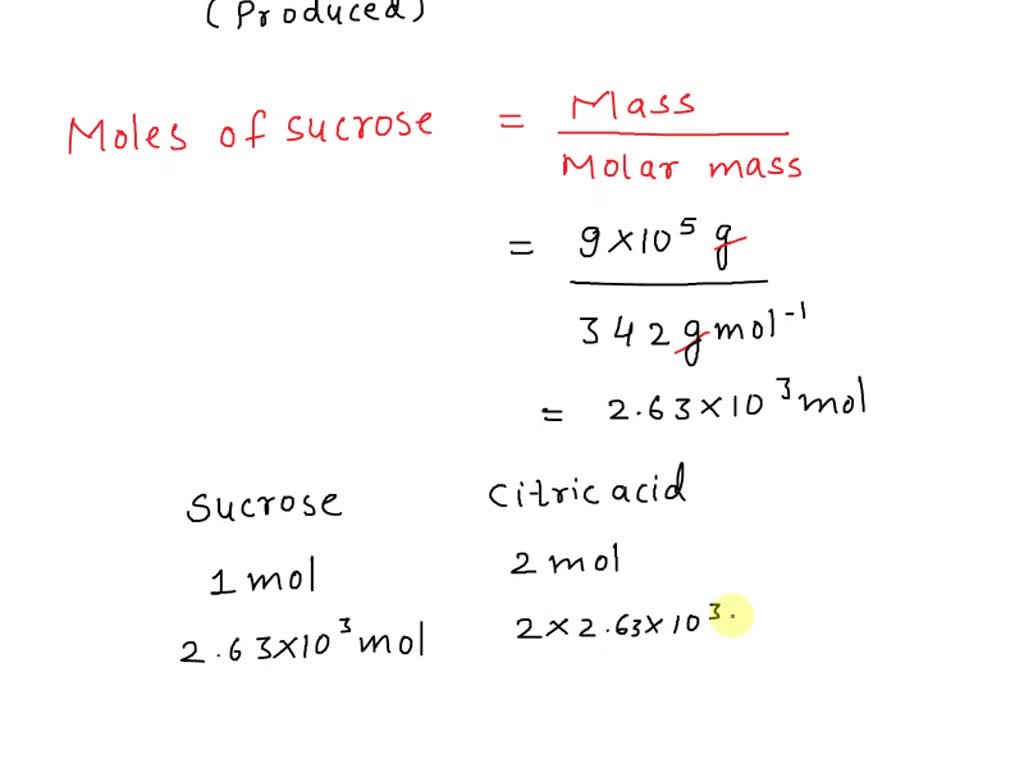
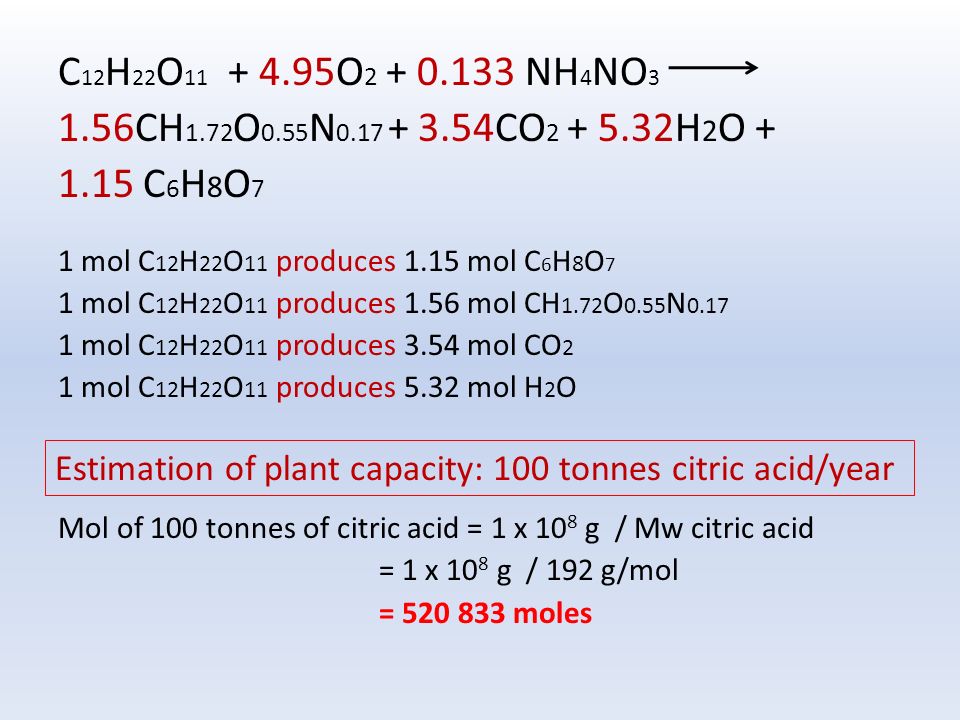
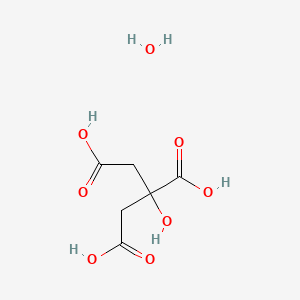
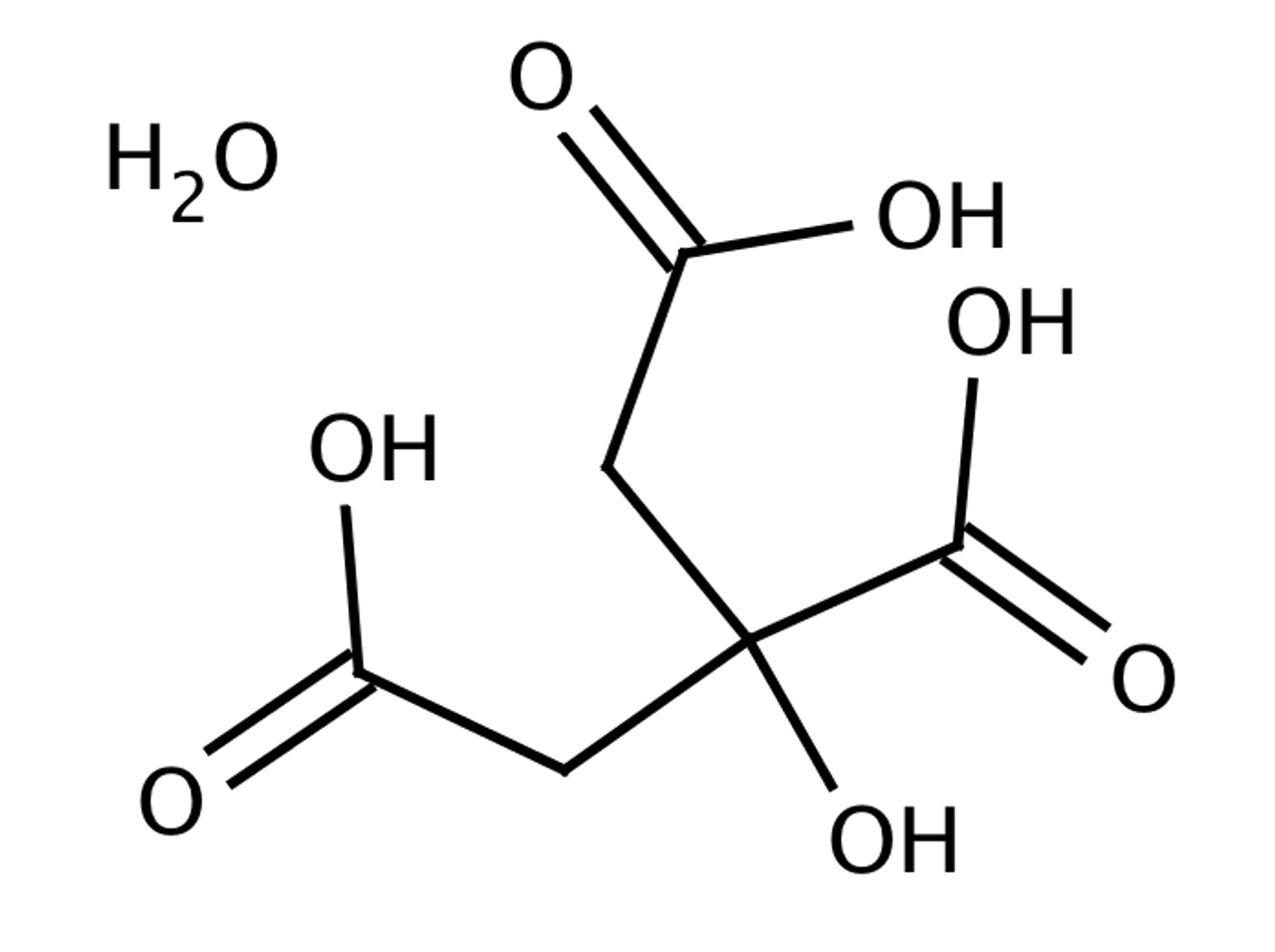
SOLVED: Citric acid ce(C6H8O7)(CX6HX8OX7) , named for its natural occurrence in citrus fruits, is produced in large quantities industrially. This is often achieved through large-scale fermentation of sucrose and/or glucose. The reaction