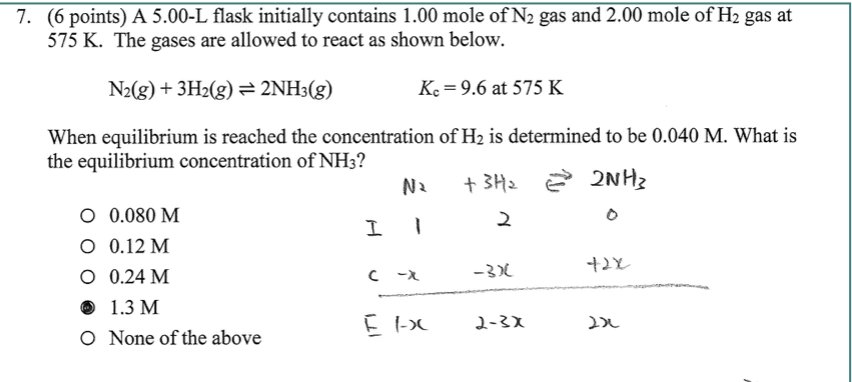
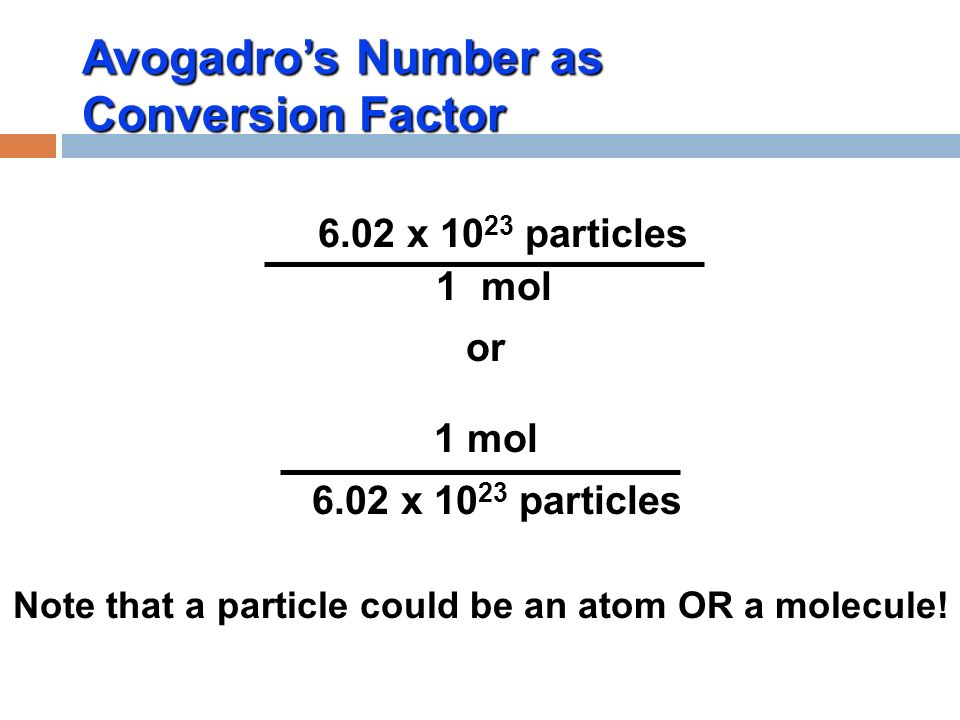
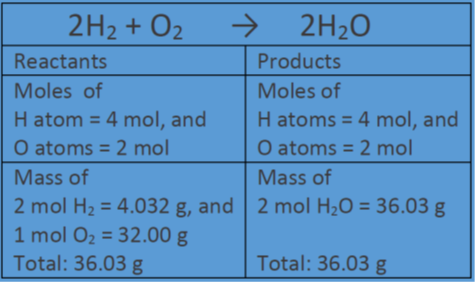
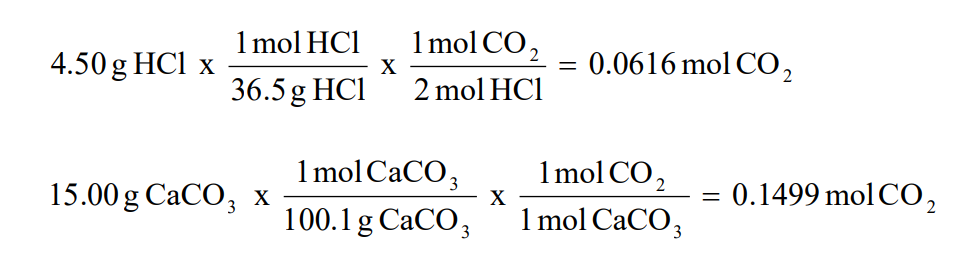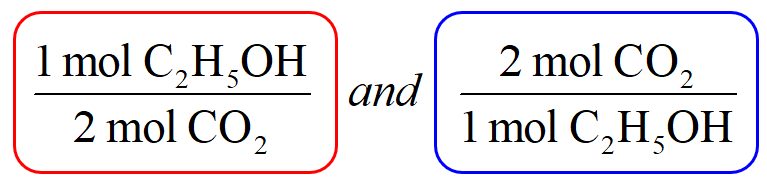THE “MOLE” A Mole (mol) is a unit of measurement used for counting very small things (atoms, molecules, etc.) A mole of something is similar to a dozen. - ppt download
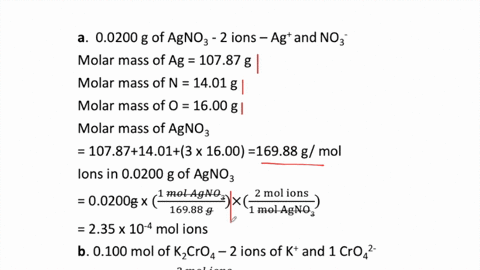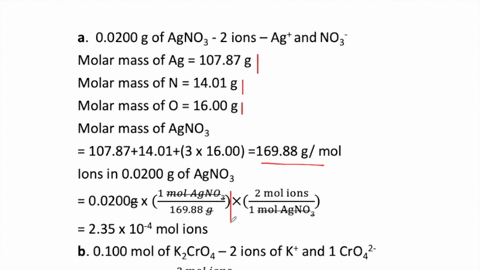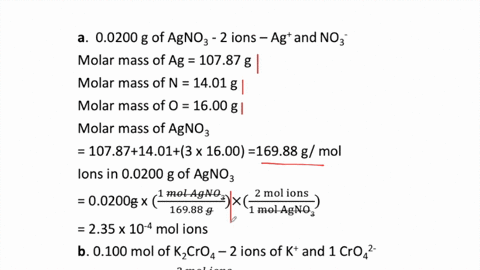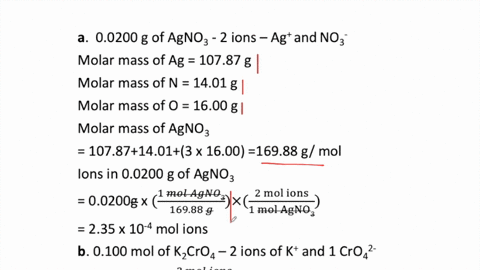
SOLVED:How many moles of ions are in each compound? a. 0.0200 g of AgNO 3 b. 0.100 mol of K2 CrO4 c. 0.500 g of Ba(OH)2 d. 1.00 ×10^-9 mol of Na2 CO3

Question Video: Calculating the Percentage by Mass of Water in Alum Given Its Chemical Formula | Nagwa
32 The dissociation of CO2 can be expressed as 2CO2 converted into 2CO +O2. If 2 mol of CO2 is taken initially and 40

How to Find Moles of Product from Moles of Reactant using a Chemical Equation | Chemistry | Study.com